Kwanan nan, StrongStep® SARS-CoV-2 Antigen Rapid Test (Professional Edition) daga Nanjing Liming Bio-products Co., Ltd. ya sami takardar shedar HSA ta Singapore, Malaysia (MDA) shawarar da aka ba da shawarar, kuma yana cikin Ma'aikatar Lafiya ta Burtaniya da Sabis na Jama'a (DHSC) sun kimanta da kansa kuma sun sami yabo.
Kafin wannan, StrongStep® SARS-CoV-2 na'urar gano antigen daga Nanjing Liming Bio-products Co., Ltd. ta sami nasarar samun takardar shedar EU CE, Cibiyar Kula da Abinci da Magunguna ta kasar Sin (NIFDC) ta tabbatar da rajistar rajistar rajista, ta shiga cikin Rockefeller. Gidauniyar ta ba da shawarar jeri, Takaddun shaida na Hukumar Tarayyar Jamus don Magunguna da Na'urorin Kiwon Lafiya (BfArM), , Takaddun shaida na Guatemala, Takaddar FDA ta Indonesiya, Takaddar Ma'aikatar Lafiya ta Italiya, Takaddar FDA ta Philippines, Takaddun shaida na HSA na Singapore, Takaddar Ecuador, Takaddar Brazil (ANVISA), Takaddun shaida na Chile , Argentina takardar shaida, Dominica takardar shaida, Guatemala takardar shaida da sauran takaddun shaida.A halin yanzu, Afirka ta Kudu, Indiya, EUL na WHO, FDA ta EUA, masu ba da izini na Turai da sauran aikace-aikacen takaddun shaida suna kan ci gaba.
Majiyar hoto: Ma'aikatar Lafiya ta Malaysia ta ba da shawarar

HOTO: Takaddar HSA ta Singapore
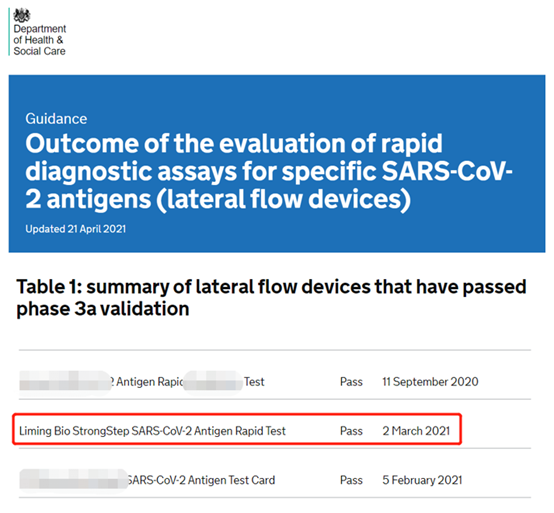
(Madogararsa ta hoto: Gidan yanar gizon DHSC na Ma'aikatar Lafiya da Ayyukan Jama'a ta Burtaniya)
A cikin 2020, Ma'aikatar Lafiya da Sabis na Jama'a a Burtaniya za ta tabbatar da tsattsauran tantance masu saurin gano cutar COVID-19 da ke shigowa cikin ƙasar don tabbatar da cewa sun isa daidai kuma abin dogaro.Akwai samfurori 120 da ke shiga cikin tsarin tabbatarwa, wanda samfurori 19 ne kawai suka wuce tabbatarwa.Bayan watanni 6 na tsauraran maimaitawa da tabbatarwa, samfurori masu inganci guda 200 da munanan samfuran marasa kyau 1,000 sun tabbatar da kyakkyawan aikin Nanjing Liming Bio-products Co., Ltd. na saurin ganowa ga COVID-19.
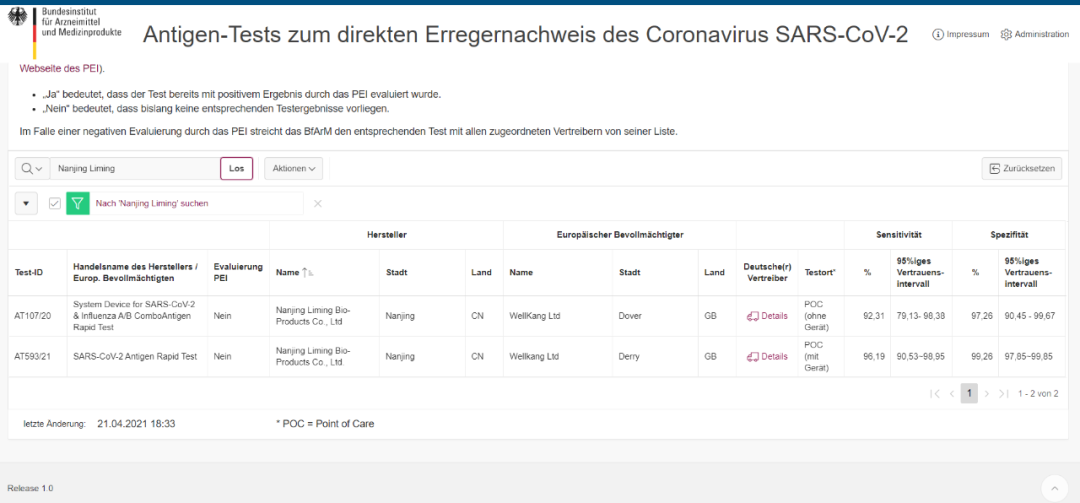
(Madogararsa ta hoto: Gidan yanar gizon Hukumar Kula da Magunguna da Na'urorin Lafiya ta Jamus (BfArM))
Gwajin takaddun shaida na hukuma: AT593/21
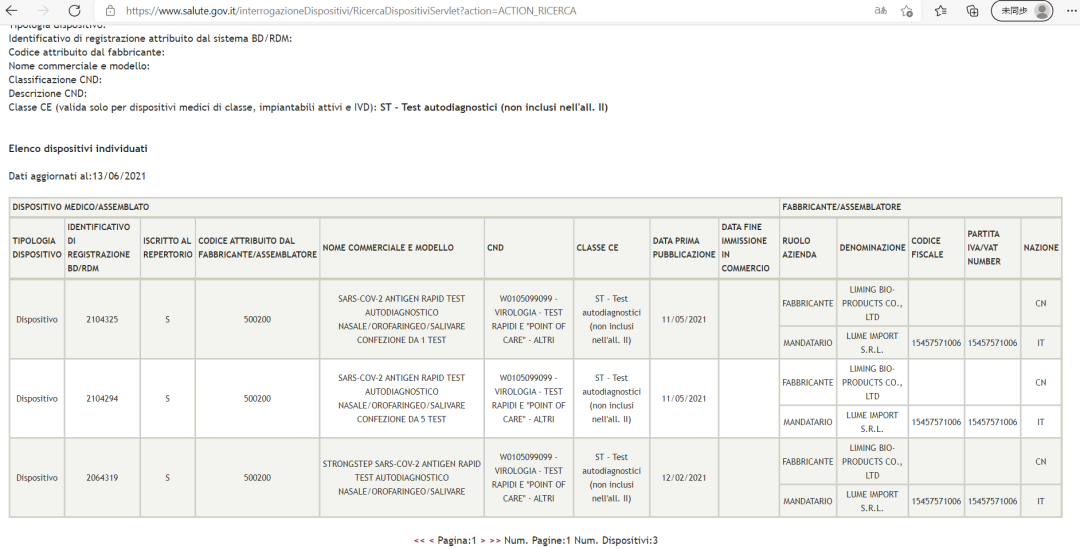
Ma'aikatar Lafiya ta Italiya ta amince da StrongStep® SARS-CoV-2 Antigen Rapid Test sigar gwajin kai-da-kai (nau'in gwajin kai)
Source: Gidan yanar gizon hukuma na Ma'aikatar Lafiya ta Italiya (Ministero della Salute)

StrongStep® SARS-CoV-2 Antigen Rapid Test ya yaba da shawarar masu amfani da Italiyanci
Gwajin antigen SARS-CoV-2 yana da sauri, daidai, mai sauƙin aiki, kuma yana buƙatar ƙananan kayan aiki da ma'aikata.Yana da matukar dacewa da saurin binciken da ake zargi da kamuwa da cutar sabon kambi mai girma, musamman don saurin ganewar cututtukan da aka tattara.Ana iya amfani da shi azaman layin farko na kariya don shawo kan annoba, ana amfani da shi don gano cututtukan da wuri, don taimakawa rigakafi da shawo kan cutar, da sarrafa yaduwar cutar.
COVID-19 zai kasance cikin yanayin annoba na dogon lokaci a nan gaba, kuma buƙatar gwaji za ta ƙaru sosai.Don yanayin aikace-aikacen daban-daban, Nanjing Liming Bio-products Co., Ltd. ya haɓaka nau'ikan abubuwan ganowa na SARS-CoV-2, "Ganewar ganowar nucleic acid SARS-CoV-2 + Ganewar antigen SARS-CoV-2 + SARS-CoV- Ganewar rigakafin 2 + SARS-CoV-2 / A da B antigen antigen sau uku gwajin sauri + SARS-CoV-2 / A da B nucleic acid gwajin sau uku + SARS-CoV-2 na dangi "Maganin cikakken yanayin ya dace da bukatun. na ganowa da rigakafi a kowane matakai a kasuwannin duniya.Taimakawa sosai don rigakafi da sarrafa cutar ta COVID-19 ta duniya da rigakafi da sarrafa cututtukan numfashi kamar mura.
Lokacin aikawa: Juni-23-2021







